For Healthcare Providers
Help your patients understand their data and engage in their health.

As access to rich health data through consumer wearables becomes commonplace, your patients may ask for help understanding the data from their Fitbit devices. You may also want to learn about how wearables can allow your patients to better track their health.
Our team of physicians with expertise in cardiology, sleep medicine, neurology and sports medicine created this resource to help you learn about key Fitbit device features and metrics to help you better serve your patients.
This page outlines wellness features as well as the indications of use of regulated features (FDA approved) particularly as it pertains to heart health in the United States. If you are outside the US, please see here for Global user manuals or contact us for more information. It is not meant to be exhaustive but rather a quick resource to give you more context.
Heart Rhythm Features
These features are clinically validated and cleared for use by notified bodies in select countries.
ECG for Heart Rhythm Assessment
What is it?
A user can acquire a 30-second single-lead electrocardiogram (ECG) and get a heart rhythm assessment of Sinus Rhythm, Atrial Fibrillation (AFib), or Inconclusive based on a FDA-cleared software algorithm (sensitivity 98.7%, specificity 100%).
How does it work?
- Fitbit Sense and Charge 5 have electrodes on the bottom and side(s) of the device so when the user places their finger(s) on the side(s) of the device the electrical signal between the right arm and left arm can be acquired, similar to Lead I of a standard ECG. The ECG signal is then analyzed by a Fitbit software algorithm for signs of Sinus Rhythm or Atrial Fibrillation, or it may report Inconclusive.
- There are 3 types of Inconclusive reports: Inconclusive: Low Heart Rate when the HR is too low (<50 bpm); Inconclusive: High Heart Rate when the HR is too high (>120 bpm); and Inconclusive: Didn’t Get A Good Reading when the ECG signal is low or noisy.
- It does not analyze for other heart rhythms. See Fitbit’s ECG App Physician’s Guide.
Indications for Use (US):
- The Fitbit ECG App is FDA-cleared (K200948) as “a software-only mobile medical application intended for use with Fitbit wrist wearable devices to create, record, store, transfer, and display a single channel electrocardiogram (ECG) qualitatively similar to a Lead I ECG. The Fitbit ECG App determines the presence of atrial fibrillation (AFib) or sinus rhythm on a classifiable waveform.
- The AFib detection feature is not recommended for users with other known arrhythmias. The Fitbit ECG App is intended for over-the-counter (OTC) use. The ECG data displayed by the Fitbit ECG App is intended for informational use only. The user is not intended to interpret or take clinical action based on the device output without consultation of a qualified healthcare professional. The ECG waveform is meant to supplement rhythm classification for the purposes of discriminating AFib from normal sinus rhythm and not intended to replace traditional methods of diagnosis or treatment. The Fitbit ECG App is not intended for use by people under 22 years old.”
Clinical Trial validation:
- The algorithm had a sensitivity and specificity for AFib of 98.7% and 100%, with 5.9% inconclusive, in a clinical trial of 421 participants. The Fitbit ECG app was tested in a clinical study of 421 people who were in sinus rhythm or AFib on 12-lead ECG. The Fitbit ECG algorithm was able to correctly identify people with AFib 98.7% of the time and correctly identify people with normal sinus rhythm 100% of the time, with 5.9% Inconclusive. [Clinicaltrials.gov: NCT04176926.]
Data Output:
- Users can review ECG results through the Fitbit app on their phone and share a PDF with their healthcare provider. Users need to install the Fitbit ECG app on their device, which includes instructions on how to acquire the 30-second ECG. The Fitbit ECG app data is in the Heart Rhythm Assessment tile within the Health Assessments & Reports section of the Fitbit app. This stores past results and allows the user to share a PDF of the ECG tracing. A Fitbit help page provides more detailed information.
Supported Fitbit devices:
- Sense 2, Sense and Charge 5. [May not be available in all countries.]
Irregular Heart Rhythm Notifications
What is it?
The Fitbit Irregular Heart Rhythm Notifications (IHRNs) feature analyzes pulse rate data during inactivity and sends a notification to the user when it detects signs of atrial fibrillation (AFib) with a of 98% positive predictive value when in a clinical trial of over 450,000 participants. Read the Fitbit Heart Study publication from the American Heart Association .
How does it work?
Fitbit wearables can have optical sensors for photoplethysmography (PPG). PPG detects the increase in blood volume in the skin with each heartbeat to detect the pulse at the wrist. Fitbit wearables also have sensors to detect motion as that can affect PPG signal quality. Pulse rate data are collected continuously and analyzed when there is minimal motion and the signal quality is good. If the IHRN software algorithm detects the pulse is irregular for at least 30 minutes of analyzable data, then an IHRN is generated.
Indications for Use (US):
- The FDA-cleared software algorithm is indicated for adults (22 or older) without a prior diagnosis of AFib while most patients develop it in their 60s, 70s, or 80s. The Fitbit Irregular Heart Rhythm Notifications feature is FDA-cleared as “a software-only mobile medical application that is intended to be used with compatible consumer wrist-worn products to analyze pulse rate data to identify episodes of irregular heart rhythms suggestive of atrial fibrillation and provide a notification to the user. The Fitbit Irregular Rhythm Notifications is intended for over-the-counter (OTC) use.
- It is not intended to provide a notification on every episode of irregular rhythm suggestive of atrial fibrillation and the absence of a notification is not intended to indicate no disease process is present; rather the Fitbit Irregular Heart Rhythm Notifications is intended to opportunistically surface a notification of possible atrial fibrillation when sufficient data are available for analysis.
- These data are only captured when the user is still. Along with the user’s risk factors, the Fitbit Irregular Heart Rhythm Notifications can be used to supplement the decision for atrial fibrillation screening but is not intended to replace traditional methods of diagnosis or treatment. The Fitbit Irregular Heart Rhythm Notifications has not been tested for and is not intended for use in people under 22 years of age. It is also not intended for use in individuals previously diagnosed with atrial fibrillation.”
Clinical Trial validation:
- The Fitbit Heart Study enrolled over 450,000 users and IHRNs occurred in 1% (4% in those over 65). For the 1,057 participants who had a subsequent 7-day ECG patch, the IHRN algorithm had a 98% positive predictive value for concurrent AF >30 seconds on ECG, which was the primary endpoint.
- The diagnostic yield for confirming AF on the 7-day ECG patch done after the IHRN was 32%. [Clinicaltrials.gov: NCT04380415]; presented at the 2021 Scientific Sessions of the American Heart Association]. Read the IHRN Study design included in the American Heart Journal.
Data Output:
- Users can review IHRNs through the Fitbit app on their phone. Users need to enroll into the Fitbit Irregular Heart Rhythm Notification feature on their device.
- The Fitbit IHRN data is in the Heart Rhythm Assessment tile within the Health Assessments & Reports section of the Fitbit app. This stores past results and allows the user to review heart rate data from past IHRNs.
Supported Fitbit devices:
- Sense, Sense 2, Versa 4, Versa 3, Versa 2, Versa Lite, Luxe, Inspire 2, Inspire 3, Charge 5, Charge 4, and Charge 3. [May not be available in all countries.]
Wellness Features
These features are not intended to inform the diagnosis or treatment of a disease.
Low and High Heart Rate Notifications
What is it?
A low or high heart rate (HR) notification is triggered when the Fitbit wearable detects a HR outside the low or high thresholds and the user appears to be inactive for 10 minutes. The HR thresholds are initially based on age and resting heart rate, but the user can customize the thresholds. Users receive a notification with the estimated HR and when it occurred, plus they can log any potential factors or symptoms.
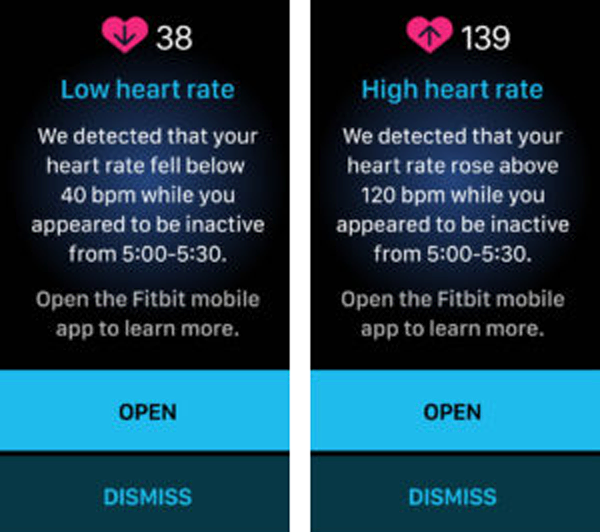
How does it work?
Fitbit wearables can have optical sensors for photoplethysmography (PPG). PPG detects the increase in blood volume in the skin with each heartbeat to detect the pulse at the wrist. Similar PPG technology is used in pulse oximetry machines to estimate HR and SpO2 . Fitbit wearables also have sensors to detect motion, which are used to determine inactivity.
Interpretation:
- HR can be low during sleep, in very fit individuals, or due to medications. It can be elevated with stress, excitement, caffeine, or illness/fever. Cardiac conditions can range from sinus bradycardia and heart block to supraventricular or ventricular tachycardias.
- There are additional issues that can affect the PPG signal and HR estimate, including motion, device fit, wrist tattoos, and cold weather (due to peripheral vasoconstriction). As PPG detects pulse rate at the wrist, not electrical HR, it can underestimate true HR during atrial fibrillation or other conditions where not every heartbeat generates a strong pulse.
Validation:
Independent studies have compared Fitbit HR measurements against ECG under a range of conditions. A 2020 study in Nature Digital Medicine by Bent et al. found the Fitbit Charge 2 had a Mean Absolute Error of 7 bpm during inactivity across a range of skin tones.
Disclaimer:
HR measurement is a wellness feature therefore low and high HR notifications are not considered diagnostic and have not been cleared by the FDA for the detection or treatment of any condition.
Sharing data with medical professional:
Users can access this data via the Health Metrics tile in the Fitbit mobile app.
Data Output:
Users can activate and adjust the thresholds for HR notifications through the Fitbit app on their Android phone or iPhone. Fitbit has a help page for users here on these and other HR features.
Supported Fitbit devices:
Sense 2, Sense, Versa 3, Versa 4, Inspire 3 and Charge 5. [May not be available in all countries.]
Oxygen Saturation: Nighttime SpO2
What is it?
The Nighttime SpO2 feature is a single point estimate of the average blood oxygen saturation across an entire session of sleep. It is derived from optical sensors shining red and infrared light on the wrist and measuring the ratio of the reflected light.
How does it work?
Certain Fitbit wearables have both red and infrared optical sensors for photoplethysmography (PPG), similar to finger-based pulse oximetry machines. The ratio of red and infrared light reflected from the wrist is related to the proportion of oxygenated hemoglobin and is used to estimate SpO2. The average estimated SpO2 during sleep and Estimated Oxygen Variation are calculated after the user wakes.
Interpretation:
Estimated Nighttime SpO2 during sleep is typically >90% but can be low due to breathing abnormalities or other health conditions, high altitude, or physical factors affecting blood flow to the wrist. Variation in blood oxygen during sleep is typically low, so high variation may indicate disturbed breathing.
Validation:
SpO2 has been tested by artificially desaturating healthy individuals and comparing to a blood co-oximeter, as well as evaluating a clinical population of COPD patients being evaluated for overnight oxygen with a fingertip pulse oximeter. Both tests resulted in Root Mean Square (RMS) errors within the ISO standard at 3 – 3.5%.
Disclaimer:
NighttimeSpO2 is a wellness feature, so these measurements are not considered diagnostic and have not been cleared by the FDA for the detection or treatment of any condition.
Sharing data with medical professional:
Users can access this data via the Health Metrics tile in the Fitbit mobile app.
Supported Fitbit devices:
Sense, Versa 3, Versa 2, Luxe, Charge 5 and Charge 4 [May not be available in all countries.]
Active Zone Minutes
What is it?
Active Zone Minutes (AZMs) allow a user to track moderate to vigorous physical activity (MVPA) and may help them reach the American Heart Association (and others’) guidelines for physical activity (150-300 minutes of moderate physical activity, or 75-150 minutes of vigorous physical activity, per week).
How does it work?
- Fitbit wearables measure motion and heart rate which together can be used to estimate relative intensity of physical activity. Per AHA guidelines, this means users earn one AZM for every minute of moderate activity (ie, fat burn zone) and two AZMs for every minute of vigorous activity (i.e. cardio or peak zone).
- Active Zone Minutes are based on accepted measures of activity intensity using Heart Rate Reserve (HRR): moderate intensity being based on 40-60% HRR and >60% representing vigorous activity.
Validation:
- The use of heart rate based exercise intensity, as opposed to questionnaires (which have recall bias) or purely movement related measures (which are inaccurate), is more physiologically valid in the context of physical activity for health. Evidence supports the use of the HRR method if heart based intensity measures, and the use of the Karvonen formula and other applied equations to predict maximum heart rate is also more accurate then the conventional 220-age (years) method.
- Historically, physical activity has been measured by standardized questionnaires and AZMs provide a more accurate measurement. However, there is recall bias and people tend to overestimate their levels of physical activity when compared to objective measures. Internal testing and independent peer-reviewed studies have shown that the Karvonen formula used in the AZM calculation is more accurate than the traditional 220-age.
Disclaimer:
It has been noted that there are some outliers in Active Zone Minutes calculations when calculated using Fitbit devices. These are most likely those >50 (especially older), and low BMI. Higher AZM numbers are seen in these groups, and this may in part relate to heart rate detection issues in some individuals.
Data Output:
- Currently, users cannot change the threshold for AZMs. Users can set and adjust exercise goals.
- Users can download a wellness report that summarizes 30 days of data.
Supported Fitbit devices:
Sense, Charge 5, Charge 4, Inspire 2, Luxe, Versa series.
Sleep Duration
What is it?

Sleep duration is the estimated amount of time an individual spent sleeping in their main sleep period. By tracking their daily sleep duration, individuals can determine if they’re allowing for at least 7 hours of sleep as recommended for adults by the American Academy of Sleep Medicine.
How does it work?
Fitbit can estimate sleep and wake from a variety of signals coming from the device, including accelerometer-derived activity levels and wrist photoplethysmography-derived heart rate patterns (such as sleep-related reductions in heart rate and increases in heart rate variability).
Interpretation:
- Consistently getting an adequate amount of sleep is not only essential for maintaining health, but is also important for ensuring optimal physical and cognitive function while awake. In 2014, the United States Centers for Disease Control and Prevention (CDC) noted that over 1/3 of the adult population does not allow for the recommended opportunity of 7 or more hours of sleep on a nightly basis, which was associated with higher rates of chronic medical conditions.
- The American Academy of Sleep Medicine [ref] and the National Sleep Foundation [ref] have both provided recommendations for the average adult to allow for at least 7 hours of sleep on a nightly basis. While 7-9 hours of nightly sleep is deemed to be adequate for most, the clinical sleep community recognizes that multiple factors, from genetics to medical conditions, contribute to what defines an adequate amount of sleep for a given individual, with most epidemiologic studies identifying a J-shaped relationship between sleep duration and health outcomes [ref]. As such, sleep durations lower or higher than the usual 7–9-hour range may be normal for an individual.
Validation:
There is evidence of significant state-specific changes in the signals used in the differentiation of sleep-wake states – notably heart rate variability (HRV) [ref] and activity [ref, ref]. Fitbit’s sleep algorithms have been extensively validated both internally and through publications from various independent researchers [ref].
Disclaimer:
While Fitbit sleep algorithms have been validated in both healthy individuals and a number of clinical populations, it is known that the algorithms may misestimate sleep in specific individuals whose physiology disrupts the signals upon which the sleep algorithms depend, such as people with sleep-related movement disorders, sleep-related breathing disorders, parasomnias, and the like. Despite this limitation, when used to monitor sleep-wake behaviors over time, algorithms are generally quite consistent in their application, allowing for the ability to interpret patterns over time. As with clinical-grade actigraphy, Fitbit is known to slightly underestimate actual sleep duration and overestimate the amount of wakefulness during the sleep period, a phenomenon that may be more pronounced in individuals with sleep-related physiologic disruptions that limit signal accuracy (as noted above).
Data Output:
Sharing data with medical professional:
Users can download a wellness report that summarizes 30 days of data.
Supported Fitbit devices:
All Fitbit devices that track heart rate (except Charge HR and Surge).
Sleep Consistency
What is it?

The times when a Fitbit user starts and ends their main sleep period can be tracked over time to determine if there is consistency or variability in their sleep schedule. By tracking their sleep patterns from day to day, users can focus on the American Academy of Sleep Medicine’s recommendation to allow adequate opportunity to get enough sleep on a regular basis.
How does it work?
Fitbit determines sleep and wake from a variety of signals coming from the device, including accelerometer-derived activity levels and wrist photoplethysmography-derived heart rate patterns (such as sleep-related reductions in heart rate and increases in heart rate variability).
Interpretation:
- In the majority of individuals, bedtime and wake time closely approximate the actual sleep onset and sleep offset times. Because the circadian rhythm relies upon regularity in the timing of the sleep period, the lower the variability (i.e.more regular the bedtime and wake time are), the stronger the circadian rhythms and the healthier the sleep patterns.
- In individuals with highly irregular sleep patterns, there is a high probability that there will be dysfunction in waking activities, caused by a misalignment between the internal biological clock (i.e., circadian rhythm) and the demands of the social schedule. This misalignment has been termed “social jet lag” because the dysfunction is similar to that experienced when physically traveling over multiple time zones. Numerous studies have highlighted the importance of a regular sleep-wake schedule in overall health and wellbeing.
Validation:
There is evidence of significant state-specific changes in the signals used in the differentiation of sleep-wake states – notably heart rate variability (HRV) [ref] and activity [ref, ref]. Fitbit’s sleep algorithms have been extensively validated both internally and through extant publications from various independent researchers [ref].
Disclaimer:
While Fitbit sleep algorithms have been validated in both healthy individuals and a number of clinical populations, it is known that the algorithms may misestimate sleep in specific individuals whose physiology disrupts the signals upon which the sleep algorithms depend, such as people with sleep-related movement disorders, sleep-related breathing disorders, parasomnias, and the like. Despite this limitation, when used to monitor sleep-wake behaviors over time, algorithms are generally quite consistent in their application, allowing for the ability to interpret patterns over time. Moreover, given the fact that Fitbit is not able to ascertain intent to sleep or wake and relies upon signals to infer this, wake time is a more reliable indicator of sleep patterns because the transition from the inactivity of sleep to the activity of wake is more likely to reflect an end to the sleep period. Comparatively, the quiescent period of wakefulness prior to sleep onset can be quite variable for individuals, therefore bedtime is a less accurate indicator of when an individual intended to start the sleep period.
Data Output:
Sharing data with medical professional:
Users can download a wellness report that summarizes 30 days of data.
Supported Fitbit devices:
All Fitbit devices that track heart rate (except Charge HR and Surge).

